Mohamad-Ali Salloum is a Pharmacist and science writer. He loves simplifying science to the general public and healthcare students through words and illustrations. When he's not working, you can usually find him in the gym, reading a book, or learning a new skill.
How do drugs pass through our cell membranes?
Share
Have you ever wondered how medicines reach their target? For example, how paracetamol goes from the guts in the digestive system to the brain passing through a lot of membranes and which are considered intact and impermeable to a lot of substances. Or how does oxygen pass from the air in the lungs into our bloodstream and then finally into the tissues and cells?
One keyword can answer these questions:
Transporters,
...well actually sometimes it doesn’t require a transporter.
To know how this happens, First, let’s discuss the membranes.
Cell membranes have two key characteristics:
1) Semi-permeability, where only certain materials may freely cross – large and charged substances are typically blocked.
2) Selectivity, where membrane proteins regulate the passage of material that cannot freely cross. These membrane proteins are called transporters.
Thus, the passage of molecules and substances across a biological membrane may occur either passively or actively.
1) Passive Transport
This type of transport is the most common mechanism of absorption for drugs. It is the passage of molecules from a high concentration location to a low concentration location (along the concentration gradient). This is just simply how nature works. And this happens so easily that it does not need the energy to do it. Thus, NO ATP hydrolysis is required. And that’s why it’s called “passive”.
OKAY, Mohamad-Ali…. we now understand that no energy is needed, but how do molecules go from one side to another?
a) Simple diffusion:
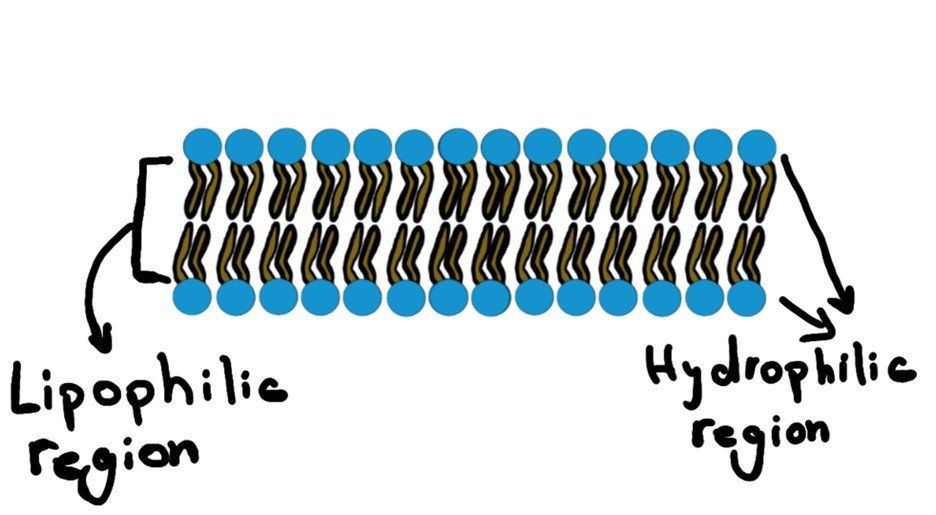
Since the core of the cellular membrane is lipophilic (Figure.1), thus, any lipophilic molecule (like ciprofloxacin) will have no problem just diffusing through the membrane without any effort.
The same happens with very small molecules like O2
and CO2.
b) Osmosis:
Passive transport of water molecules (H2O) from the low concentration to the high concentration of solute. This usually happens since the cellular membrane will not allow the solute to pass through it and thus water will always try to equalize the concentration of a solute across the membrane. Not to mention that water can pass easily through the membrane.
c) Facilitated Diffusion:
Small charged molecules (i.e. ions) and large molecules (i.e. sucrose) will not be able to pass easily like the lipophilic or very small molecules. They will need some help, but not energy. They just need a tunnel to pass through. AND who provides these tunnel services?
You guessed it right…
the
MEMBRANE PROTEINS, where they will help these molecules to pass through the lipophilic cellular membranes.
Examples:
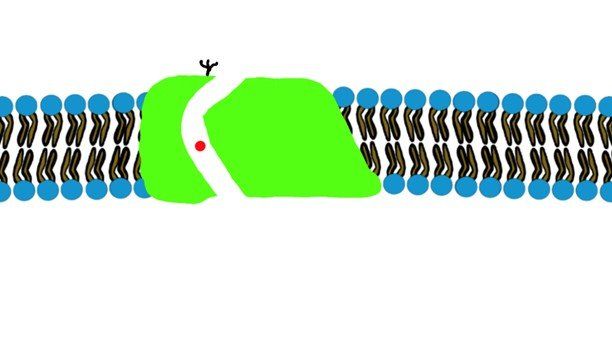
- Ions will be able to pass through their ion channels. ( Sodium, Potassium, Calcium) (Fig.2)
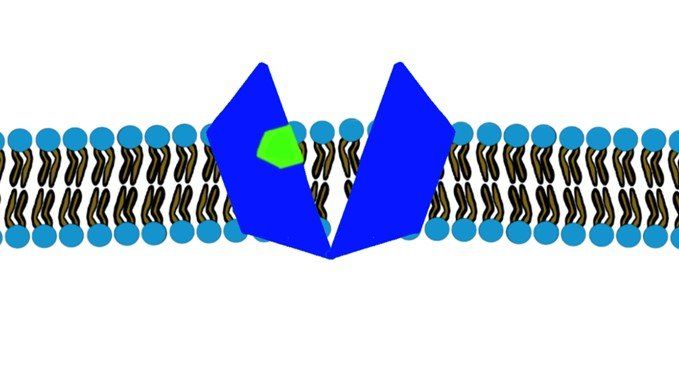
- Large molecules will have their carrier proteins. (Glucose)(Fig.3)
d) Paracellular Transport:
In blood vessels, paracellular passage of solutes and fluid through intercellular gaps is sufficiently large that passive transfer across the endothelium of capillaries and postcapillary venules is generally limited by blood flow. Capillaries of the CNS and a variety of epithelial tissues have tight junctions that limit the paracellular movement of drugs. Water-soluble compounds can use this transport method, like glucose and amino acids.
2) Active Transport
When you jump into the pool, you don’t need energy to go down (only gravity is needed). But if you wanted to go up, you need energy to go up again to the jumping point.
Now let’s downscale this example into molecules.
Nature allows the movement of molecules along their concentration gradient with no energy needed. BUT, what if the molecules are required to move against their concentration gradient? (from the lower concentration to the higher concentration side)
Cells use different energy sources to allow this active transport through their membranes.
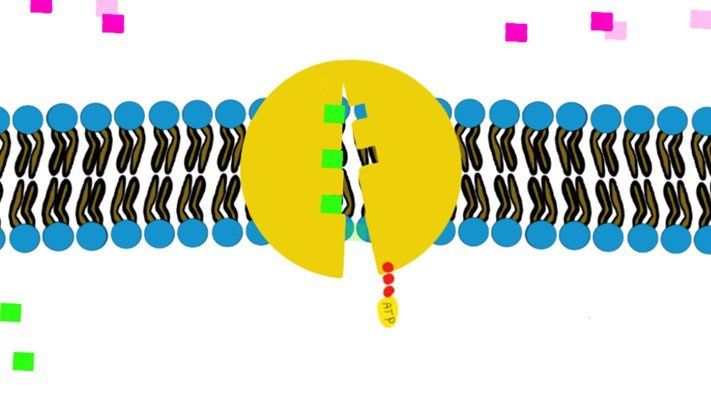
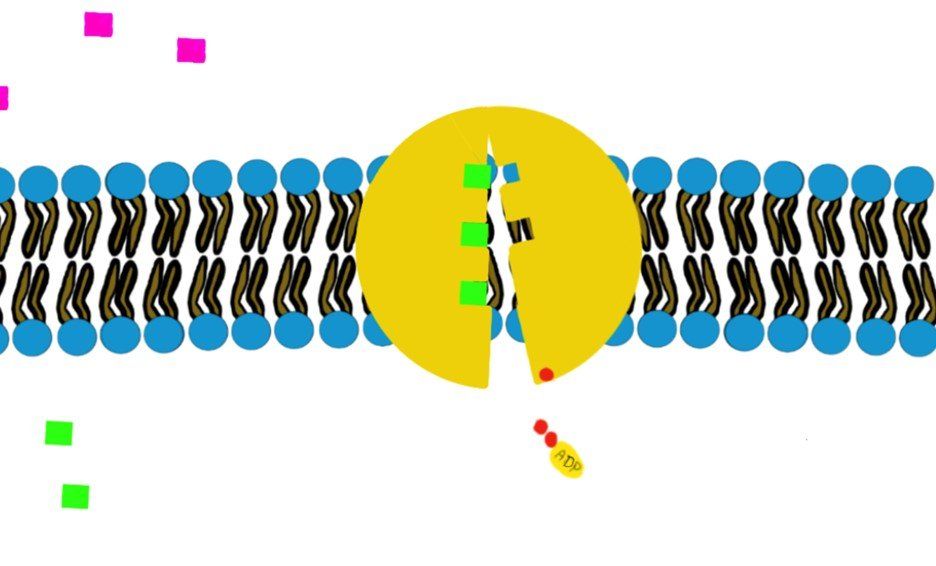
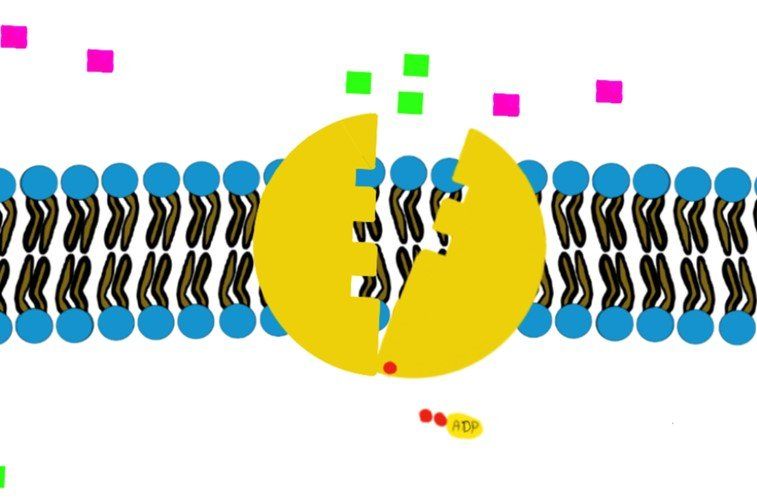
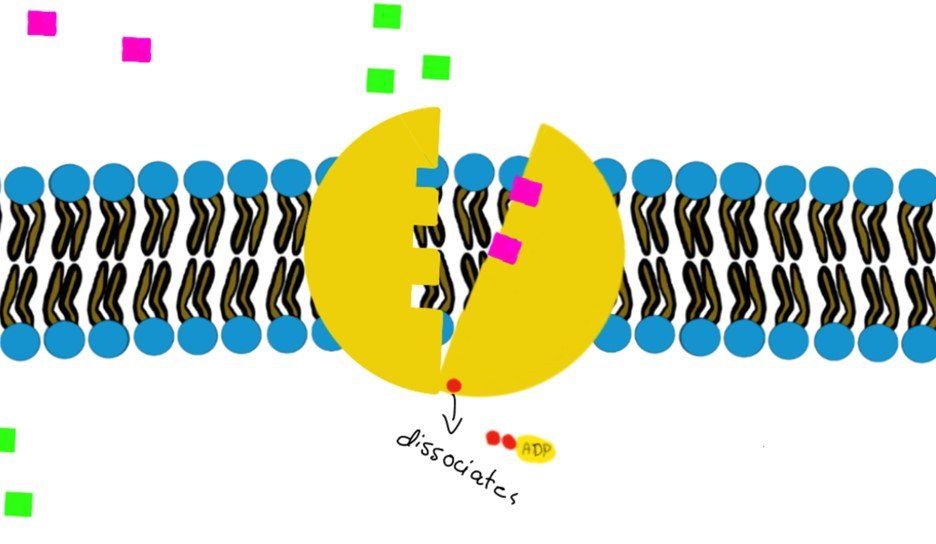
a) Primary (direct) active transport:
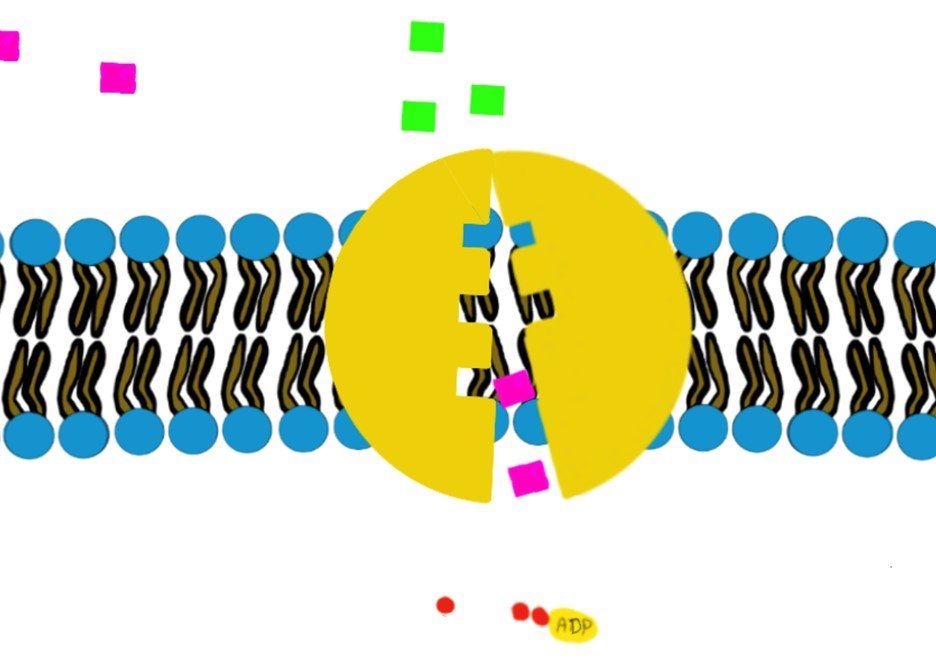
It involves hydrolysis of ATP to provide energy, like the Na+/K+-ATPase.
How?
Swipe through the pictures below and read the caption for more details.
Some transporters move just one molecule against their gradient (i.e. uniport transport) and other transporters can move 2 molecules against their gradient. (Co-transport through Na+/K+-ATPase)
b) Secondary (indirect) active transport:
You know that we can gain energy from the waterfall, right?
The energy that the falling water creates can be converted to electricity that we can use.
That’s how secondary active transport works.
A molecule that is moving from its high concentration to the low concentration will not require energy, however, the energy that it creates with this movement will be utilized to move another molecule from its low concentration to its higher concentration side.
Scientifically speaking, this type of active transport includes the coupling of the required molecule A with another molecule B that is moving “along” its electrochemical gradient.
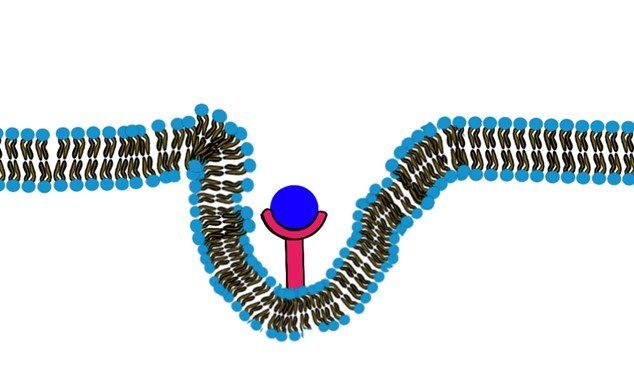
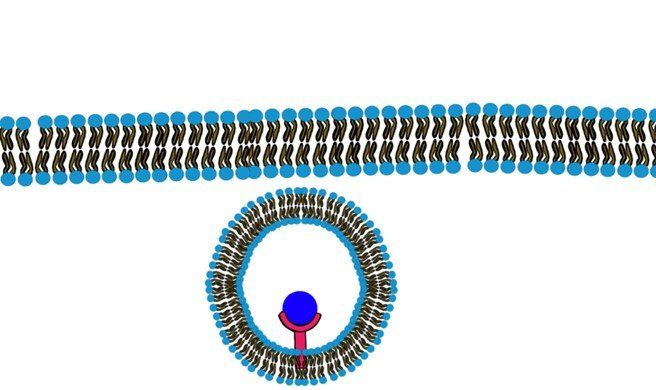
c) Endocytosis:
When the molecules are very large and they are lipid insoluble such that they will not be able to pass through the membrane or any channel that facilitates the passage, they will bind to a specific receptor on the cell membrane. This binding will trigger the formation of a pocket in the cell membrane and eventually pinching down and the formation of an intracellular vesicle that contains the molecule. (Fig.9, Fig.10) (Swipe to see both Figures.)
d) Pinocytosis:
It literally means “cell drinking”. The difference between Pinocytosis and endocytosis is that Endocytosis requires binding to a receptor to trigger the process.
Pinocytosis is the process of ingestion of extracellular fluids where molecules close to the membrane will be ingested too. It is considered an active transport since it requires energy.
Summary:
| Transport Method | Active/Passive | Material Transported |
|---|---|---|
| Simple Diffusion | Passive | Small molecular weight material |
| Osmosis | Passive | Water |
| Facilitated Transport/ Diffusion | Passive | Sodium, Potassium, Calcium, Glucose |
| Paracellular Transport | Passive | Water-soluble compounds like Glucose and Amin acids |
| Primary Active Transport | Active | Sodium, Potassium, Calcium |
| Secondary Active Transport | Active | Amino Acids, Lactose |
| Receptor-mediated endocytosis | Active | Large quantities of macromolecules |
| Pinocytosis | Active | Small molecules (they are ingested alongside with water |
Check out below our animated video about the transporters!
Resources:
1) Bertram G. Katzung, Marieke Kruidering-Hall, Anthony J. Trevor - Katzung & Trevor’s Pharmacology Examination and Board Review-McGraw-Hill Education (2019)
2) Goodman & Gilman 13e
List of Services
ABOUT THE AUTHOR
Mohamad-Ali Salloum, PharmD
Share
Recent articles: