Mohamad-Ali Salloum is a Pharmacist and science writer. He loves simplifying science to the general public and healthcare students through words and illustrations. When he's not working, you can usually find him in the gym, reading a book, or learning a new skill.
CAR T-Cell Therapy: What do you need to know about it?
Share
Briefly speaking, it is the use of one’s immune cells- specifically the T-cells- then teach them how to detect cancer cells in our bodies and kill them. It sounds simple, but talking about how to do it, here comes the challenge.
Is this technique approved by FDA?
Is it efficacious? And why is it important to develop?
How did the scientists isolate the T-Cells?
Can it kill both solid and liquid tumors?
How do they teach these cells to detect cancer cells?
How do they allow the cells to proliferate and then inject them back into our bodies in the effort of reducing the complications (e.g., Cytokine Release Syndrome (CRS))?
A LOT OF QUESTIONS can arise, but this technique was not developed in a single night. It was developed over decades and its complexity increases with the more we know. In this article, I am going to talk about the key points that will make you knowledgeable about this technique.
Is this method FDA approved?
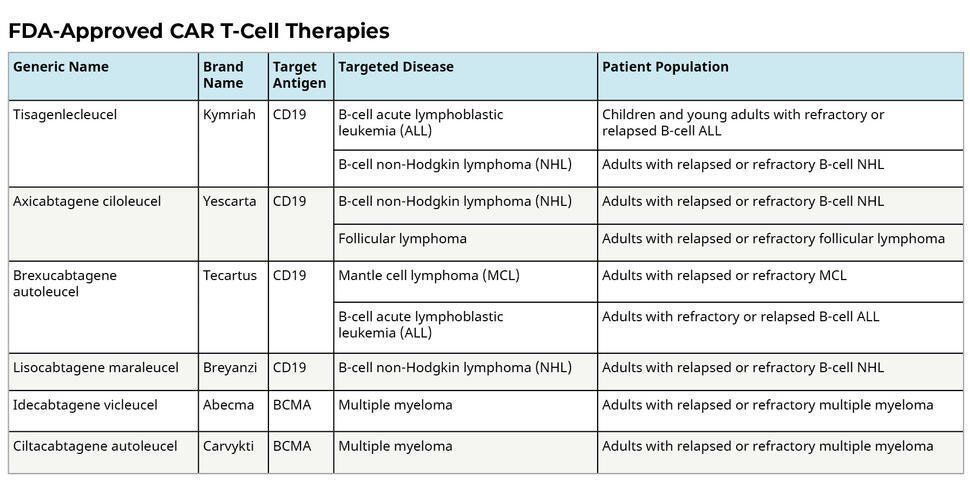
Since 2017, Six CAR-T-cell-based therapies were approved by the FDA (1):
- Axicabtagene ciloleucel, approved on 30 August 2017.
- Tisagenlecleucel, approved on 01 May 2018.
- Brexucabtagene autoleucel
- Lisocabtagene maraleucel
- Idecabtagene vicleucel
- Ciltacabtagene autoleucel
After the conduction of several Phase I and Phase II clinical trials, these medications were approved by FDA for the treatment of the below hematological malignancies:
Why is this method significant?
What empowers this method is its ability to treat patients with relapsed and refractory diseases where previous treatments were not enough to eliminate cancer.
As we know, more than 80% of children diagnosed with Acute Lymphoblastic Leukemia (ALL) that arises in B-cells will be cured by intensive chemotherapy. But limited effective treatments are available for those whose cancers return after initial treatment (1). Providing effective treatment with CAR T-cell therapy for these cases is such a blessing!
What is its efficacy?
Large Phase I/II clinical trials at several centers confirmed the high level of efficacy of CAR T cells, particularly in Acute Lymphoblastic Leukemia where complete remission (CR) (the disappearance of all signs of cancer) rates of 70 to 93% are achieved. (2)
How do they work?
White blood cells called T cells are taken from the patient's blood and are modified in the lab by adding a gene for a receptor (called a Chimeric Antigen Receptor or CAR), which helps the T cells attach to a specific cancer cell antigen. The CAR T-cells are then given back to the patient where they will detect the cancer cells and help in eliminating them. I will explain in more scientific detail how they work in a future article.
What are the side effects?
Like all medications, this type of therapy will also cause some side effects, and sometimes these side effects may be life-threatening.
1) Cytokine release syndrome (CRS):
It is the most common one and may lead to serious adverse events. This happens because when CAR T-cells multiply, they will release large amounts of cytokines into the blood and this will hyperactivate the immune system and will lead to (3):
- High fever and chills
- Trouble breathing
- Severe nausea, vomiting, and/or diarrhea
- Feeling dizzy or lightheaded
- Headaches
- Fast heartbeat
- Feeling very tired
- Muscle and/or joint pain
2) Nervous System problems:
- Headaches
- Changes in consciousness
- Confusion or agitation
- Seizures
- Shaking or twitching (tremors)
- Trouble speaking and understanding
- Loss of balance
3) Allergic reactions during the infusion
4) Abnormal levels of minerals in the blood, such as low potassium, sodium, or phosphorous levels
5) A weakened immune system, with an increased risk of serious infections
6) Low blood cell counts, which can increase the risk of infections, fatigue, and bruising or bleeding
Are the side effects manageable?
An article was published in January 2021 discussing the most recent management notes for minimizing adverse effects of CAR T-cell therapy, and they are:
- Continuous close monitoring of the patients,
- Rapid detection of the side effect occurrence,
- Accurate intervention with supportive care, anti-cytokine or corticosteroid therapy.
Thus, prophylactic and/or preventive strategies to avoid toxicities without affecting efficacy and predictors of toxicities should be identified in later studies. (4)
What is the downside of this therapy?
Although a lot of promises are built on this therapy, however, in a study on patients with diffuse large B-cell lymphoma, the scientists observed long-term survival in less than half of the patients treated and that in select clinical scenarios alternate therapies (standard treatment) may be as efficacious as CAR T therapy. Not to mention their extremely high cost, where the most recently approved CAR T-Cell treatment is worth ~450,000$. Thus, more randomized and prospective clinical trials should be conducted to have more clear results. (2) (5)
Can this therapy be used in solid tumors?
CAR T-cell therapy can be effectively used in blood tumors since, for example, in B-cell cancers there are antigens homogeneously expressed (e.g., CD19) on the surface of the cancer cells, and the therapy will be able to identify the cancer cells easily and eliminate them.
Unfortunately, in solid tumors (e.g., lung, prostate, breast cancers…) there is no homogenous expression of antigens on the surface of the cancer cells, and thus the CAR T-Cell therapy will not be able to differentiate between the normal and cancer cells which will lead to its failure as a treatment. (2)
The scientists had an idea and thought that if there is no homogeneity of antigens, then they can target several antigens at the same time. This can be used to prevent the cancer cells from escaping the re-programmed T-cells (6). Another method was developed in a pre-clinical model where CAR T cells secrete IL-12 and lead to the activation of macrophages that mediated the elimination of antigen-negative tumor cells. (7)
A review was done in December 2021 about the current clinical trials using CAR T-cell therapy in solid tumors and they concluded that an extensive amount of ongoing research will be necessary to assess the safety and feasibility of their place in treatment in solid tumors and maybe the success of the ongoing Phase I clinical trials will lead to the conduction of Phase II and Phase III studies in the future. (8)
List of Services
ABOUT THE AUTHOR
Mohamad-Ali Salloum, PharmD
Share
Recent articles: