Pharmacy & Medicine
Recent articles
Cardiology
Microbiology

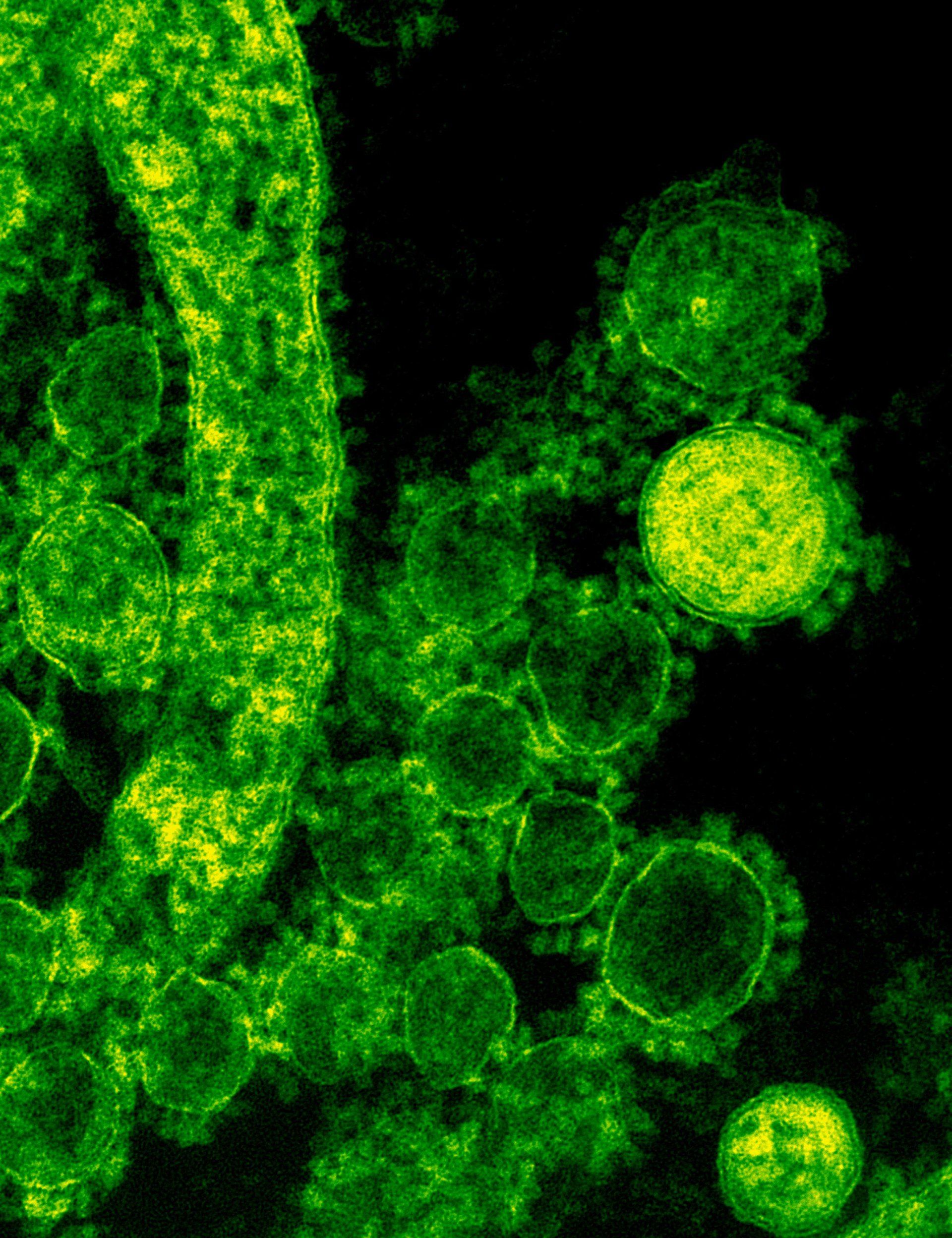
On June 1, 2022, WHO (World Health Organization) stated that More than 550 monkeypox cases have been identified in at least 30 countries. First of all, What is monkeypox? I t is a zoonotic viral disease native to Africa. Cases usually occur near tropical rainforests, where animals that carry the virus live. Cases in the U.S. are rare and associated with international travel from places where the disease is more common. Zoonotic means that it can spread between species and in this case from- animals to humans. I t is a member of a virus family called orthopoxvirus . It has 2 distinct strains: The Central African (Congo Basin) strain. The West African strain. The Central African strain is known to spread more easily and cause more severe symptoms . What are the symptoms? Symptoms of the disease include: Skin lesions, Fever, Body aches. Chills Swollen lymph nodes Exhaustion The symptoms are similar to smallpox but milder . Severe cases are seen in patients with immune deficiencies and young children . Incubation period , the time from infection to onset of symptoms, can range from 5 to 21 days. The illness typically resolves within 2 to 4 weeks. Is it fatal? In recent times, the case fatality ratio of monkeypox is around 3-6%. How is it transmitted? Although transmission is limited between Humans, it can happen: Through close skin contact, Bodily fluids, Virus-contaminated objects. In contrast to what was being spread about its transmission method, specialists say that the disease is not an STD (sexually-transmitted disease), however, it can spread via direct contact during sex. Scientists still lack a lot of data, and that’s why they still can’t confirm how long it’s been spreading. It’s important to mention that it is thought by WHO that this virus is unlikely to outbreak and turn into a global pandemic like the COVID-19 virus. But again, they are not sure. Treatments that can be used? Usually symptoms go away on their own within few weeks, however, some individuals require treatment for recovery. “Tecovirimat (TPOXX), as both oral and IV medication is approved in the U.S. for treating smallpox and oral form in Europe to treat cowpox, monkeypox, and smallpox. The FDA approved brincidofovir (Tembexa) in 2021 to treat smallpox and it was tried with Monkeypox, however, it had poor efficacy. Is there a vaccine? There is an approved vaccine for monkeypox and it is called MVA-BN. Unfortunately, it is not widely available. This vaccine is also known as Imvamune, Imvanex or Jynneos) and was approved in 2019 for use in preventing monkeypox. Even though people who were vaccinated against smallpox will have a certain level of protection against monkeypox, they need to take precautions to protect themselves and the others.
Remdesivir.. I bet you’ve heard about this molecule during the pandemic. This molecule was originally approved by FDA in October 2020 for hospitalized patients aged 12 and above. (Their weight should be at least 40 kilograms) Properties of Remdesivir: This molecule is an antiviral drug that acts by limiting the SARS-CoV-2 replication. How? The virus has a machine that can use the human’s cell components to multiply itself. This machine is called the RNA-dependent RNA polymerase. Remdesivir is a nucleotide prodrug of an adenosine analog. This means that it mimics the function of another molecule that is used by the machine to build the virus. When Remdesivir sits in place of the molecule that was supposed to be in its place, it terminates the RNA transcription prematurely, thus preventing the virus from multiplying itself. The usual Adverse events that were observed include: nausea, Elevated transaminase levels, Increase in prothrombin time without a change in the international normalized ratio (INR), and hypersensitivity. Approvals: Intravenous Remdesivir is approved for treatment of: - Mild to moderate Covid-19 in high-risk, non-hospitalized patients (a 3-day course is initiated within 7 days of symptom onset.) - Hospitalized patients with Covid-19 (a 5-day course) FDA expanded the approval for the intravenous Remdesivir where It’s now available as FDA Emergency Use Authorization (EUA) for treatment of COVID-19 in non hospitalized and hospitalized pediatric patients: - Weighing 3.5 kg to 40 kg - Age <12 years and weighing > 3.5 kg. - Children as young as 28 days can be given the medication. A phase II/III study was done by “Gilead Sciences” and the scientists observed that 75% of the pediatric patients had clinical improvement at day 10, and 85% showed improvement at last assessment. Adverse events were reported in 72% of the children, and 21 % of them had serious adverse event. The FDA eventually assessed that the safety profile of Remdesivir is similar in pediatric subjects and adults. Adverse events that were observed in pediatric patients: Elevated levels of liver enzymes, allergic reactions, fever, shortness of breath, rash, nausea, sweating, or shivering. (2)

The molecule is called “Sabizabulin”, and the manufacturer is called “Veru”, an oncology biopharmaceutical company that is based in USA. This medication was being tested in moderate and severe COVID-19 cases, and it worked! The results show that it has the potential to cut the virus’s mortality rate by more than 50%. Note that the Mortality rate is a measure of the number of deaths in a particular population per unit of time. Usually in clinical trials, the study team perform an interim analysis, which is a statistical analysis done on the data at some point during the study to see if they continue with the trial or put it down. If the results are positive, the clinical trial continues recruiting more patients to reach its target. If the results are negative, the clinical trial may be terminated if it is clear that the study drug is not superior to the standard therapy/placebo. This study was randomized, double-blinded, placebo-controlled phase 3 Covid-19 clinical trial. Patients in both treatment groups were allowed to receive standard of care including remdesivir, dexamethasone, anti-IL6 receptor antibodies, and JAK inhibitors. Placebo group (n=52) had a 45% mortality rate compared to the sabizabulin-treated group (n=98) which had a 20% mortality rate. The interim analysis results were so good that it was unethical to continue giving the patients a placebo. An Independent Data Management Committee (IDMC) recommended the study to be stopped early due to overwhelming evidence of efficacy. The article published stated that Sabizabulin showed statistically and clinically meaningful 55% reduction in deaths compared to Placebo in Moderate-Severe hospitalized patients (p=0.0029). Regarding its safety profile, the medication was well tolerated, and no safety concerns were identified. The next steps is that Veru will meet with FDA to seek Emergency Use Authorization. This means that the medication may be available in the market in the next few months. Gary Barnette, PhD, Chief Scientific Officer of Very has stated that “What makes these findings more relevant is that the pharmacological activity of sabizabulin is independent of COVID-19 variant type.”
Oncology
Briefly speaking, it is the use of one’s immune cells- specifically the T-cells- then teach them how to detect cancer cells in our bodies and kill them. It sounds simple, but talking about how to do it, here comes the challenge. Is this technique approved by FDA? Is it efficacious? And why is it important to develop? How did the scientists isolate the T-Cells? Can it kill both solid and liquid tumors? How do they teach these cells to detect cancer cells? How do they allow the cells to proliferate and then inject them back into our bodies in the effort of reducing the complications (e.g., Cytokine Release Syndrome (CRS))? A LOT OF QUESTIONS can arise, but this technique was not developed in a single night. It was developed over decades and its complexity increases with the more we know. In this article, I am going to talk about the key points that will make you knowledgeable about this technique. Is this method FDA approved? Since 2017, Six CAR-T-cell-based therapies were approved by the FDA (1): - Axicabtagene ciloleucel, approved on 30 August 2017. - Tisagenlecleucel, approved on 01 May 2018. - Brexucabtagene autoleucel - Lisocabtagene maraleucel - Idecabtagene vicleucel - Ciltacabtagene autoleucel After the conduction of several Phase I and Phase II clinical trials, these medications were approved by FDA for the treatment of the below hematological malignancies:

12 patients took the treatment with Dostarlimab and their rectal cancer vanished. Just like that💫 The physicians did physical exams, digital rectal examinations, biopsies, PET scans, and MRIs and they found nothing! Not to mention that no adverse events of grade 3 or higher have been reported!! How was the idea of this study formulated? Usually, oncologists will prescribe to patients with locally advanced rectal cancer neoadjuvant chemotherapy and radiation (neoadjuvant treatment is the kind of treatment given before doing the surgery to help shrink the tumor size and prevent its further spread). For the patients suffering from metastatic rectal cancer due to a deficiency in mismatch repairing, positive results were observed when PD-1 blockers were used. Researchers hypothesized that there will be better results when this type of medication is used when the rectal cancer is still local and did not metastasize. What is the design of the study? So, this Phase 2, prospective study was initiated, and the participants received a dose of Dostarlimab every three weeks for six months, and the plan was to undergo standard treatments of chemotherapy, radiation therapy, and then surgery following the treatment with Dostarlimab. However, researchers found that in every case, the cancer was cleared through the experimental treatment alone. This result is considered astonishing although it’s only in a small number of patients because it’s never happened in the history of cancer. What is Dostarlimab-gxly? Dostarlimab-gxly is a humanized monoclonal antibody that works as a PD-1 blocker, AKA checkpoint inhibitor. It binds to the PD-1 receptor and prevents its interaction with its ligands, PD-L1 and PD-L2. Like Pembrolizumab, It releases the brakes on an immune cell, freeing it to recognize and attack cancer cells, according to MSK. Its side effects include (but not limited to): - Fatigue/asthenia, - Nausea, - Diarrhea, - Anemia, - Constipation. For this medication to prove itself, a longer follow-up is needed to assess the duration of response (DoR). Also, more studies should be done on a larger sample because 12 patients showing that the tumor vanished is not enough to prove that a molecule or a drug is effective 100%. Note that this drug is being also investigated if it can beat other cancers like gastric, prostate, and pancreatic cancers.
Pharmacology

The U.S. Food and Drug Administration (FDA) plays a crucial role in ensuring the safety and efficacy of medications. Despite rigorous testing, some drugs are recalled post-approval due to unforeseen adverse effects. This essay explores ten such medications, detailing the reasons behind their recalls.

Anaphylaxis is a severe, life-threatening allergic reaction that can occur rapidly, requiring immediate medical intervention. Traditionally, epinephrine autoinjectors have been the standard of care for such emergencies. However, the recent approval by the U.S. Food and Drug Administration (FDA) of a novel nasal spray, named neffy, marks a significant milestone in the management of anaphylactic reactions
Understanding Pharmacogenomics: The world of pharmacology is continually evolving, and one of the most exciting frontiers is Pharmacogenomics. Usually, we treat patients based on their clinical characteristics. But with pharmacogenomics, we take into consideration the genetic variability of drug metabolism and response within each person. This allows us to exactly treat what the patient is suffering from, and at the same time reduce some of the unwanted side effects in some cases.
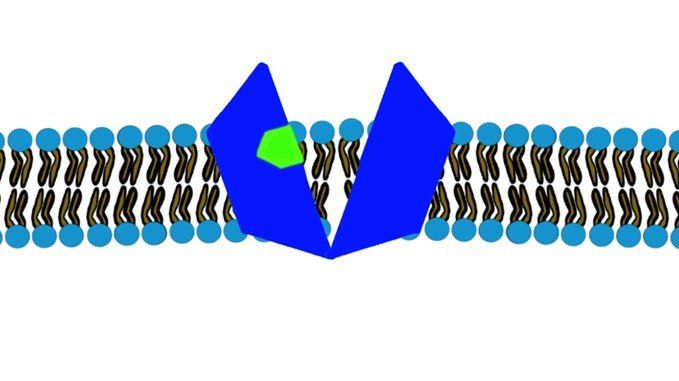
Have you ever wondered how medicines reach their target? For example, how paracetamol goes from the guts in the digestive system to the brain passing through a lot of membranes and which are considered intact and impermeable to a lot of substances. Or how does oxygen pass from the air in the lungs into our bloodstream and then finally into the tissues and cells? One keyword can answer these questions: Transporters, ...well actually sometimes it doesn’t require a transporter. To know how this happens, First, let’s discuss the membranes. Cell membranes have two key characteristics: 1) Semi-permeability , where only certain materials may freely cross – large and charged substances are typically blocked. 2) Selectivity , where membrane proteins regulate the passage of material that cannot freely cross. These membrane proteins are called transporters. Thus, the passage of molecules and substances across a biological membrane may occur either passively or actively. 1) Passive Transport This type of transport is the most common mechanism of absorption for drugs. It is the passage of molecules from a high concentration location to a low concentration location (along the concentration gradient). This is just simply how nature works. And this happens so easily that it does not need the energy to do it. Thus, NO ATP hydrolysis is required. And that’s why it’s called “passive”. OKAY, Mohamad-Ali…. we now understand that no energy is needed, but how do molecules go from one side to another? a) Simple diffusion: Since the core of the cellular membrane is lipophilic (Figure.1), thus, any lipophilic molecule (like ciprofloxacin) will have no problem just diffusing through the membrane without any effort. The same happens with very small molecules like O 2 and CO 2.
Taking a lot of medications can confuse the patients over time since they might not remember if they took the drug today or not. Not to mention that a lot of these patients are from the elderly population. David P. Wagner received his patent for inventing the pill organizer on 4 Aug 1964 . The reason he had this idea is that his wife was forgetting if she had already taken her medications. This invention helped a lot of patients to remember taking their medications on time every day but the question we are asking today is that “Are they safe?” Studies on Medication organization devices (MODs) are of poor quality, and the relationship between adherence and health outcomes is unclear. (2) Heat, air, light, and moisture may impact the effectiveness and safety of a medicine. The original container is designed to protect the medicine from these elements. (3)Some medicines cannot be repacked and must be stored in the original container until it is time for the patient to take them. (3) A study done in 2013 showed that the use of the organizers for storing and carrying tablets and capsules exposes these medications to environmental factors from which their original packaging protected them, compromising their stability and safeness. (1) Older people who switch to using pill organizers could experience adverse effects and even hospitalization -according to research from the University of East Anglia. New research reveals that people who switch from usual medication packaging to a pill organizer are more likely to become unwell than those not using them. When these patients were switched to a pill organizer and suddenly started taking more of their prescribed medication than previously, they experienced normal side effects of the medication." (2) In Conclusion, the research team says that patients should consult their General Physician or Pharmacist before switching to a pill organizer.

Pharmacology is the study of medications, and it is divided into two major branches: - Pharmacodynamics: How medications affect our body. - Pharmacokinetics: How our body affects the medications. It can be also branched into many other sub-branches (pharmaco-toxicology, pharmacogenomics, pharmacogenetics, pharmacognosy, pharmaco-economics…..). At the first glance, it may seem a very hard subject to study, especially when you see the daunting amount of medications that we have to memorize and know their details. When you start studying it, you will realize it is really hard..no cap. During my collage days, I realized this early on and wanted to master this hellish subject and pass it. The advantage of studying from the below references is that you will master pharmacology. The downside is that IT TAKES A LOT OF TIME. Here is a list of the references that helped me to understand this subject. 1) Bertram G. Katzung, Marieke Kruidering-Hall, Anthony J. Trevor - Katzung & Trevor’s Pharmacology Examination and Board Review-McGraw-Hill Education (Edition 13) In my opinion this is the reference that every healthcare professional (HCP) should start from when studying pharmacology. What makes it special is that it is not too much detailed and at the same time it’s not too much briefed. This will help you to understand Pharmacology without going too deep where a beginner might get confused. 2) Symptoms in the Pharmacy A Guide to the Management of Common Illnesses by Alison Blenkinsopp , Martin Duerden , John Blenkinsopp This reference is the one you should go for when doing the internship in the community pharmacy. It’s important to do it during the internship since you will be able to associate the cases you are observing in the pharmacy with the information you are studying in the reference. This will allow you to memorize the information for longer time. 3) Case files. pharmacology by Toy, Eugene C. This reference contains 56 clinical cases with USMLE-style questions. It will explain pharmacology briefly and will make you revise the chapters that you’ve studied in the previous references and highlight on the main points. It will teach you how to approach clinical problems. 4) Pharmacotherapy Handbook, Eleventh Edition 11th Edition by Terry Schwinghammer In the previous three references you were studying pharmacology per drug class (opioids, analgesics, anti-inflammatory medications...). In the pharmacotherapy handbook you will understand the management of every disease and what drug what medications to give and WHY. This reference will talk about a disease and how you will manage it with the most recently approved therapies. While studying you will realize that this handbook is rich in valuable clinical information. 5) Farideh Javid, Janice McCurrie - Clinical Physiology and Pharmacology_ The Essentials (2009, Wiley) This reference will fill the gaps that were formed in your brain when you were studying pharmacology from the previous references. It will help the student to put the facts that he/she learned before into the deep memory. Testing your knowledge when solving clinical case studies will make you do connections between several chapters because a case study will describe a patient that has several comorbidities and that is taking medications from several classes. This will expose the students brain to more complex situations. 6) Goodman & Gilman Edition 13 This reference is literally a heaven of Information for health care professionals . It will provide you with everything you need to know about Pharmacology from A to Z. However, I don’t recommend to starting studying from it because it will confuse you more than it can benefit you. This reference is more recommended for healthcare professionals that want to go into depth in the chapters.

As discussed in previous article, an agonist is a molecule that causes a physiologic response when it binds to its receptor. A drug can be classified as Full agonist or Partial agonist depending on its efficacy. Let’s Say Drug A is the Full agonist and Drug B is the partial agonist. So, how both can be agonists and have different magnitude of action? A full agonist can be effective 100% although not all receptors are occupied . This means that Drug A needs less than 100% of the receptors to result in full response. On the other hand, drug B, a partial agonist will never be able to reach 100% even if it occupied 100% of the receptors present. This happens because the physico-chemical properties of the full agonist that allow it to make a bond with the receptor in a way that it can excite only 3 receptors (theoretically) to result in 100% of the effect. The physico-chemical properties of the partial agonist allow it to bind to its receptor, however, with lower ability to excite it and produce the desired response, even with occupying 100% of the receptors present. Examples of full agonists are morphine, opium and phenylephrine. Buprenorphine and Tolazoline are examples of a partial agonist.

Starting from 30 June 2015, the FDA implemented a new labelling rule for medications used during pregnancy and lactation. As discussed in the previous article, the Categories published in 1979 (A, B, C, D, and X) were causing confusion for the patients and are increasing the risk to be misinterpreted by the health care professionals. The new format is called the “Pregnancy and Lactation Labelling Rule” and is abbreviated as PLLR. Using PLLR will remove some of the uncertainty that was caused by the previous five categories since it will contain a narrative information about the medication that will describe the potential risks of drug exposure based on available, evidence-based data. Ultimately, the new format will assist the health care professionals to assess the benefit versus risk when counseling pregnant women and nursing mothers who need the medication, and thus leading to enhanced protection of the mother and her baby. After the PLLR was implemented, every pharmaceutical company that wants to register it’s new drug in the FDA, It should use the template provided by the FDA to include the required information in the leaflet. Regarding the medications that were approved by FDA after 30 June 2001, The manufacturer was given from two to four years after 2015 to change the information on the leaflet as required. Meanwhile, for the medications that were approved by the FDA before 30 June 2001, the format of the information was not required to be changed, however, It was mandated to remove the categorization that was assigned to this medication. The deadline for this action was 30 June 2018. Concerning the OTC drugs, medications that can be given without a prescription, they were not affected by the new rule. Not only the letter categorization was removed, the FDA also required the manufacturer to modify the sections in the leaflet. The below picture will illustrate the modification on sections done between the 2 formats.

Being pregnant brings to women so many questions. The questions can range from stuff related to how their bodies are affected, what should they eat, how much exercise should be done...etc.
All the questions are important to answer and make it clearer for the pregnant woman to prevent unnecessary bad things from happening. However, one of the most important questions to be asked is “What are the medications that can be administered during pregnancy and what are the medications to be avoided?”

An Antagonist is a molecule that binds to a certain receptor and LITERALLY does nothing. It just sits there, preventing an agonist from binding to perform its usual action. [1] Just like an irritating sibling... However, an antagonist has several ways to annoy the agonist and prevent it from binding to its receptor or do its effect. Let’s explore them... 1) Reversible Competitive antagonists: It is the most common and usually it sits in place of the agonist. Increasing the concentration of the agonist in the presence of the reversible antagonist will allow its binding to the receptor. And that’s why it is called “Competitive”. There is a competition between the agonist and the antagonist on who will bind to the same receptor, and if the agonist concentration was increased, it will be able to replace the antagonist. Example of a competitive antagonist is naloxone, which is used in emergencies to reverse the life-threatening effects of a known or suspected opiate (narcotic) overdose [2]. Naloxone injection can be also used after surgery to reverse the effects of opiates given during surgery. [3] 2) Irreversible Competitive antagonist: In this case, increasing the concentration of the agonist in the presence of the irreversible antagonist will have no effect. This happens because the antagonist binds irreversibly to the receptor and there is no concentration from the agonist that can replace it. Note that this kind of antagonism is also called “Competitive” since both molecules compete on the same receptor, however, the irreversible antagonist contain reactive groups that create covalent bonds with the receptor. Usually, these are used as experimental tools to investigate about the receptor function, and few are used clinically. However, irreversible enzyme inhibitors that act similarly are clinically used and include drugs such as aspirin, omeprazole, and monoamine oxidase inhibitors (MAOIs) [4] 3) Allosteric (non-competitive) antagonists: Allosteric antagonist means that this molecule does not sit necessarily on the receptor itself, but it binds near it and causes some modifications that will prevent the agonist from binding to is receptor, because simply the agonist can’t recognize the receptor (scientifically, we say that the allosteric antagonists reduce the affinity of the agonist toward their receptors). Thus, increasing the concentration of the agonist will have no effect. Example for allosteric antagonist is Ticagrelor, an anti-platelet drug. [5] 4) Physiologic antagonist: The antagonist binds to a receptor that totally differs from the one that the agonist binds on. The physiologic response that results from the binding of the antagonist will antagonize the effect of the agonist. (Even if this physiologic antagonist is an agonist itself) For example, histamine acts on Histamine Receptors of the parietal cells of the gastric mucosa to stimulate acid secretion, while omeprazole blocks this effect by inhibiting the proton pump. [4] 5) Chemical Antagonist: This antagonist will work directly on the agonist itself and not on the receptor (most of the times). The main mechanism here is the binding of this chemical antagonist to the agonist and preventing its binding to its receptors. Examples include the use of chelating agents. (e.g., dimercaprol) that bind to heavy metals and consequently reduce their toxicity. Infliximab, a neutralizing antibody, has an anti-inflammatory action due to its ability to sequester the inflammatory cytokine tumor necrosis factor (TNF). [4] 6) Pharmacokinetic antagonist: It is the ‘antagonist’ that effectively reduces the concentration of the active drug at its site of action. As we know, the Pharmacokinetic steps are Absorption, Distribution, Metabolism, and Excretion. At every step, if a drug can alter the concentration of the active drug at its site of action, it is considered a pharmacokinetic antagonist. For example, Phenytoin (anti-seizure drug) enhances the hepatic metabolism of warfarin (anti-coagulant drug), which will result in the reduction of the anti-coagulant effect. On the other hand, the rate of absorption of the active drug from the gastrointestinal tract may be reduced, or the rate of renal excretion may be increased. Interactions of this kind are common and can be important in clinical practice. 7) Partial agonists: These types of molecules, although they are agonists, however, they act as antagonists in the presence of the full agonists (refer to this article to understand difference between full and partial agonists) . [4] Example of a partial agonist is Tolazoline and when it is combined with the full agonist Phenylephrine, it acts as an antagonist.
Research

Introduction: In the realms of quality control and regulatory compliance, the terms “audit” and “inspection” are often used interchangeably. However, they represent distinct processes with unique objectives, methodologies, and outcomes. Understanding these differences is crucial for organizations aiming to maintain high standards and ensure compliance with regulations. 1.Definitions: Audit: An audit is a systematic, independent, and documented process for obtaining objective evidence and evaluating it to determine the extent to which audit criteria are fulfilled . Audits are comprehensive reviews of processes, systems, or organizations to assess their accuracy, efficiency, and compliance with established standards. Inspection: An inspection is the process of examining, measuring, and testing to determine whether an item or activity conforms to specified requirements2 . Inspections are typically more focused and specific, often involving physical examination and testing of products, equipment, or facilities. 2. Purpose: Audit: Primarily aims to improve processes by identifying weaknesses and opportunities for improvement . Inspection: Focuses on ensuring that products or services meet specified standards and requirements . 3.Scope: Audit: Broad in scope, covering various aspects of an organization, including financial, operational, and compliance areas . Inspection: Narrower in scope, typically limited to specific products, equipment, or processes . 4.Depth of Review: Audit: Involves a deep and thorough review of processes and systems . Inspection: Limited to checking conformity to specified requirements . 5.Formality: Audit: A formal and documented process with detailed reports and recommendations . Inspection: Can be less formal, with reports varying in detail . 6.Frequency: Audit: Conducted periodically, such as annually or quarterly . Inspection: Can be scheduled or unscheduled, depending on regulatory requirements . 7.Independence: Audit: Performed by internal or external auditors who are independent of the area being audited . Inspection: Can be performed by internal staff or external agencies, with varying levels of independence .